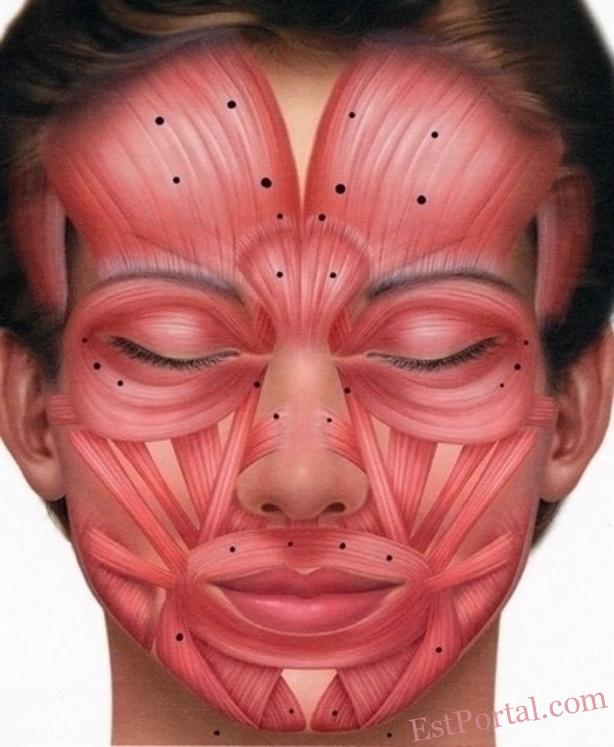
Informed Consent for Botox Injection
Article under preparation
This article is currently available only in Russian. You can switch the language in the top language bar to view the Russian version, or check back soon — the English translation will be available shortly.