Baumann Skin Type Indicator: 16 Skin Types & Care
In the early 1900s, cosmetics entrepreneur Helena Rubinstein claimed that dry, oily, combination, or sensitive were the best words to label what could be considered the four fundamental types of skin. For the ensuing century, these categories have been used to characterize skin types with only minor, if any, modifications. A modern development is the Baumann Skin Type Indicator, which offers a more detailed method to categorize skin. During the same time period, the skin care product market has developed into a multibillion dollar industry featuring numerous innovations and frequent new product introductions. The industry has, in recent years, also witnessed the emergence of ‘‘cosmeceuticals,’’ a new product category that refers to cosmetic products that may impart some biologic function to the skin.
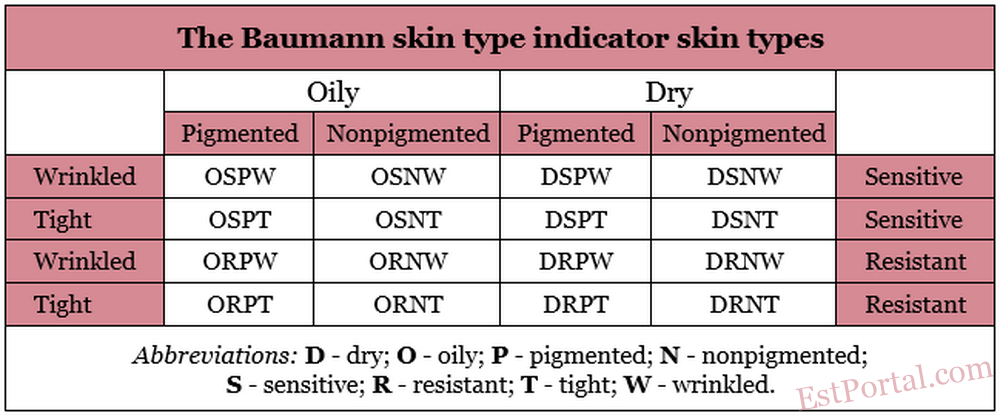
Amidst a market now deluged with a plethora of skin care products, the traditional designations for skin types have been seen as incomplete or inadequate descriptions of skin, thus providing insufficient guidance for practitioners and consumers to select the most suitable products. A more thorough depiction of skin type could yield such assistance to patients/consumers and physicians, particularly because some products are now marketed based on the skin types for which they are designed. But does a person have simply dry or sensitive skin? The skin types identified by Rubinstein tell only a fraction of the story. An innovative approach to classifying skin type, the Baumann Skin Type Indicator (BSTI), treats two of Rubinstein’s categories as one of four dichotomous parameters to characterize facial skin types: dry or oily; sensitive or resistant; pigmented or nonpigmented; and wrinkled or unwrinkled (tight). Evaluating skin based on all four parameters yields 16 potential skin-type permutations. The BSTI is a 64-item questionnaire that is designed to determine baseline skin type identifications and assessments after significant life changes. [1]
All four parameters must be considered for patients to accurately self-assess their skin type or for practitioners to be able to make appropriate skin care recommendations to their patients. For example, a person who has dry, sensitive, pigmented, wrinkled skin would require markedly different skin care products or treatments than an individual who has oily, resistant, nonpigmented, unwrinkled skin.
This article describes the four parameters that make up the BSTI, focusing on basic science and defining characteristics and summarizing the 16 skin-type variations (Table 1). Variability is a key concept underlying the questionnaire and accurately identifying skin type. Skin types are not necessarily static. Moving to a different climate or experiencing marked stress fluctuations, pregnancy, menopause, or other significant exogenous and endogenous events can engender skin type changes. Significantly, noninvasive, primarily topical therapies are the focus of treatments based on the BSTI system.
Table 1
16 skin types classified by Leslie Baumann:
- DRNT – dry, resistant, nonpigmented, tight.
- DRNW – dry, resistant, nonpigmented, wrinkled.
- DRPT: dry, resistant, pigmented, tight.
- DRPW – dry, resistant, pigmented, wrinkled.
- DSNT – dry, sensitive, nonpigmented, tight.
- DSNW – dry, sensitive, nonpigmented, wrinkled.
- DSPT – dry, sensitive, pigmented, tight.
- DSPW – dry, sensitive, pigmented, wrinkled.
- ORNT – oily, resistant, nonpigmented, tight.
- ORNW – oily, resistant, nonpigmented, wrinkled.
- ORPT – oily, resistant, pigmented, tight.
- ORPW – oily, resistant, pigmented, wrinkled.
- OSNT – oily, sensitive, nonpigmented, tight.
- OSNW – oily, sensitive, nonpigmented, wrinkled.
- OSPT – oily, sensitive, pigmented, tight.
- OSPW – oily, sensitive, pigmented, wrinkled.
Skin hydration
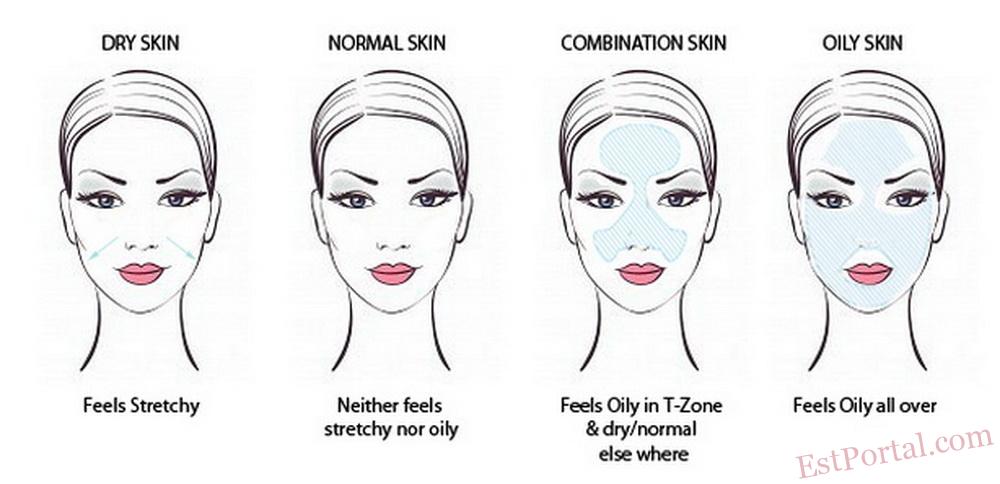
Oily Versus Dry
Having skin that is sufficiently hydrated, which would fall in the middle of the oily–dry spectrum, is most often ideal regarding this parameter. The dry end of this dichotomy is considered more troublesome than the oily end, however. Dry skin, also known as xerosis, is the result of a convoluted, multifactorial cause, but its description is relatively straightforward. Dry skin is characterized by dull color (typically gray white), rough texture, and an elevated number of ridges. [2] Levels of stratum corneum lipids, sebum, natural moisturizing factor, and aquaporin are considered to be the most important factors that regulate the degree of, or contribute to, dry skin.
Of these factors, the role of the stratum corneum (SC), especially its capacity to maintain skin hydration, is the most significant factor in the mechanism of xerosis. In turn, the SC is composed of ceramides, fatty acids, and cholesterol, among other less active constituents. When present in the proper amount and balance, these three groups of primary constituents of the SC contribute to protecting the skin and keeping it watertight. SC equilibrium is also believed to be maintained through stimulation of keratinocyte lipid synthesis and keratinocyte proliferation by primary cytokines. [3]
Improper balance in these constituents contributes to a cascade of interrelated events, including a diminished capacity to maintain water and increased vulnerability to external factors, which increases sensitivity of the SC. Xerosis results through such impairment in the SC. These flaws in the skin barrier lead to increases in transepidermal water loss (TEWL). The enzymes necessary for desmosome metabolism are inhibited by insufficient hydration, resulting in the abnormal desquamation of corneocytes. [4] Superficial SC desmoglein I levels simultaneously remain high. The resultant compromised desquamation yields a visible collection of keratinocytes manifesting in skin that is rough and dry in appearance. [5] A perturbation in the lipid bilayer of the SC because of increased fatty acid levels and decreased ceramide levels is also associated with dry skin. [6] The lipid bilayer is also susceptible to being influenced or inhibited by exogenous factors, such as ultraviolet radiation, detergents, acetone, chlorine, and prolonged water exposure or immersion. Recent research has indicated that local changes in pH may explain the initial cohesion and ultimate desquamation of corneocytes from the surface of the SC. It is believed that these changes selectively activate several extracellular proteases in a pH-dependent fashion. [7]
Natural moisturizing factor (NMF), an intracellular, hygroscopic compound found only in the SC that is released by lamellar bodies and synthesized by way of the breakdown of the protein filaggrin, plays an important role in maintaining water within skin cells. Filaggrin, which consists of lactic acid, urea, citrate, and sugars, is broken down by a cytosolic protease into free amino acids, such as arginine, glutamine (glutamic acid), and histidine, in the stratum compactum, an outer layer of the SC. [8] These water-soluble compounds stay in the keratinocytes and bind strongly to water molecules. The pace of filaggrin decomposition and the level of NMF present are attributed to aspartate protease (cathepsin). [9] Changes in external humidity can influence cathepsin, potentially yielding fluctuations in NMF production. NMF production typically increases over the course of several days after an individual enters a low-humidity environment. [10] Low levels of NMF are associated with xerosis and ichthyosis vulgaris. NMF development can be inhibited by ultraviolet radiation and surfactants. There are no products or procedures yet available to artificially regulate NMF production.
Aquaporin-3 (AQP3) is an important member in the family of homologous integral membrane proteins that selectively facilitate the transport of water and small neutral solutes, such as glycerol and urea, across biologic membranes. [11] AQP3 is present in the kidney collecting ducts and epidermis, and in the urinary, respiratory, and digestive tracts. This water channel protein that ultimately influences skin hydration is a member of a subclass of aquaporins known as aquaglyceroporins, which transport water, glycerol, urea, and other small solutes. In 2002 AQP3 was demonstrated to be expressed abundantly in the plasma membrane of human epidermal keratinocytes. [12] It is believed that the water conduction function in the skin occurs along an osmotic gradient beneath the SC, where high AQP3-mediated water permeability is displayed. AQP3 water clamps viable epidermal layers to facilitate the hydration of skin layers below the SC.
A high concentration of solutes (Na+, K+, and Cl–) and a low concentration of water (13%-35%) [13] are present in the superficial SC and produce the steady-state gradients of solutes and water from the skin surface to the viable epidermal keratinocytes. [14-16] Although transepithelial fluid transport has been studied extensively in kidneys and lungs, the molecular mechanisms of fluid transport across epidermal keratinocyte layers have not been clearly elucidated. Likewise, the relationship between keratinocyte fluid transport and SC hydration is not well understood. It is believed, however, that AQP3 improves transepidermal water permeability to shield the SC from water evaporating from the skin surface or to disperse water gradients throughout the epidermal keratinocyte layer. [12] In a study assessing the functional expression of AQP3 in human skin, investigators found that, consistent with AQP3 involvement, the water permeability of human epidermal keratinocytes was hindered by mercurials and low pH. [12] In a different study, some of the same researchers investigated skin phenotype in transgenic mice lacking AQP3 and found significantly lower water and glycerol permeability in the AQP3 null mice, buttressing previous evidence that AQP3 acts as a plasma membrane water/glycerol transporter in the epidermis. [17] Conductance measurements showed substantially lower SC water content in most cutaneous areas of the null mice. Epidermal cell water permeability is not a significant determinant of SC hydration, however, because water transport across AQP3 is slower in skin compared with other tissues. [18] The activity of AQP3 has only been shown to be enhanced through the use of extracts of the herb Ajuga turkestanica. [19] A high-end line of skin care products includes A. turkestanica as an active ingredient. In the future, skin conditions caused by excess or diminished hydration may be treated through pharmacologically manipulating AQP3.
Sebum, the oily secretion of the sebaceous glands that contains wax esters, sterol esters, cholesterol, di- and triglycerides, and squalene, [20] confers an oily quality to the skin and contributes significantly to the development of acne. In addition, sebum, which is an important source of vitamin E, is believed to provide cutaneous protection from environmental factors, whereas low levels of sebum have been cited as a potential contributing factor to dry skin development. [21] This theory has not found support, though, because low sebaceous gland activity has not been demonstrated to promote the development of xerosis. Sebum production has actually been found to play a more convoluted role in the cause of this condition. Previously, it has been speculated that sebum has no impact on epidermal permeability barrier function primarily because skin with few sebaceous glands (eg, as in prepubertal children) displays normal basal barrier function. [22] Prepubertal children between 2 and 9 years old frequently present with eczematous patches (pityriasis alba) on the face and trunk that do not emerge with the onset of sebaceous gland activity. The pharmacologic involution of sebaceous glands with supraphysiologic isotretinoin doses does not affect barrier function or SC lamellar membranes. [23-25] Similarly, using ether to denude the skin does not interrupt SC function.
Although barrier function is not influenced by sebum levels, sebum may still contribute to the etiologic pathway of xerosis in individuals who have dry, resistant skin (the DR type in the BSTI). Lipids from meibomian glands, which are modified sebaceous glands located in the eyes, are known to stave off dryness by preventing the evaporation of tears. [26, 27] Similarly, perhaps, sebum-derived fats may produce a lipid film over the skin surface, thereby preventing TEWL. A recent study evaluating permeability barrier homeostasis and SC hydration in asebia J1 mice with sebaceous gland hypoplasia supports this theory. [28] The normal barrier function in these sebum-deficient mice was attributed to consistent levels of the three most important barrier lipids (ceramides, free sterols, and free fatty acids) and the persistence of normal SC extracellular membranes. The investigators observed, however, that the asebia J1 mice exhibited diminished SC hydration, suggesting that although an intact intercellular membrane bilayer system suffices for permeability barrier homeostasis, it does not necessarily contribute to normal SC hydration. The researchers found that topically applying glycerol restored normal SC hydration. In normal skin, sebaceous gland–derived triglycerides are hydrolyzed to glycerol before transport to the skin surface. In individuals who are sebum deficient, xerosis may be allayed by replacing this glycerol. The acceleration of SC recovery has also been shown to be successful with the use of glycerol. [29]
Reduced sebum production is rarely the source of patients’ complaints, but elevated sebum production, rendering oily skin that can lead to acne, is a common complaint. The age-related trajectory of sebum production is well known. Sebum levels are typically low during childhood, increase in the middle to late teens, and remain relatively stable for decades until decreasing in the seventh and eighth decades as endogenous androgen production declines. [30] Other factors also have an impact on the level of sebum production. One’s genetic background, diet, stress levels, and hormone levels affect sebum production. A fascinating study of 20 pairs each of identical and nonidentical like-sex twins revealed nearly equivalent sebum excretion rates with significantly divergent acne severity in the identical twins, but significant differences in both parameters among the nonidentical twins, suggesting that both genetic factors and environmental factors had an impact on acne development. [31] The use of oral retinoids to shrink sebaceous glands is well established, but topical retinoids have not yet been shown to have this capacity. In addition, no other topical formulations have been demonstrated to reduce sebum production.
Skin Care for the Oily–Dry Parameter
Skin that falls in the middle of the oily–dry continuum can be best characterized as manifesting an intact SC and barrier, normal levels of NMF and hyaluronic acid (HA), normal AQP3 expression, and balanced sebum secretion. Whether or not acne develops from it, elevated sebum secretion is usually responsible for placing skin on the oily side of the oily–dry spectrum. The BSTI profile for oily skin accompanied by acne is OS, because acne-infiltrated skin is distinguished by heightened sensitivity (see later discussion). For individuals who have OS skin, treatment should focus on reducing sebum levels with retinoids, eliminating or decreasing skin bacteria with antibiotics, benzoyl peroxide, or other antimicrobials, and using anti-inflammatory ingredients. Treatment of oily skin without acne — an oily, resistant (OR) type in the BSTI — should be tailored to reduce sebum production, unless other parameters, such as dyspigmentation and wrinkling, are factors (see following sections). Sebum secretion has been effectively decreased with the use of oral ketoconazole and oral retinoids, [32, 33] but such results have not yet been seen with topical products. The sebum in OR skin can also be camouflaged using sebum-absorbing polymers and talcs.
Dry skin chronically exposed to the sun is likely characterized by an impaired skin barrier and reduced NMF. Therapy for such skin should focus on skin barrier repair and reducing sun exposure, avoiding the sun if possible or at least providing adequate sun protection. All patients who have xerosis should abstain from using harsh foaming detergents (found in laundry and dish cleansers along with body and facial cleansers), which remove hydrating lipids and NMF from the skin. Protracted bathing, particularly in hot or chlorinated water, should also be avoided by all patients who have dry skin (Box 1). For those who have very dry skin, humidifiers should be used in low-humidity environments and moisturizers should be applied two to three times daily and after bathing.
Box 1
Treatment suggestions for dry skin
How to treat dry skin
1. Preserve and replace skin lipids
- Replace them in the proper ratio
2. Prevent loss of NMF
3. Increase function of AQP3
- Glucosamine supplementation
Preserve and replace skin by
Avoiding:
- Detergents
- Prolonged water immersion, especially in chlorine and hard water
- Vigorously foaming cleansers
- Surfactants (which may deposit fatty acids on the skin)
Using
- Moisturizers containing fatty acids, ceramides, and cholesterol
- For dry, resistant (DR) skin: Look for moisturizers containing Ajuga turkestanica
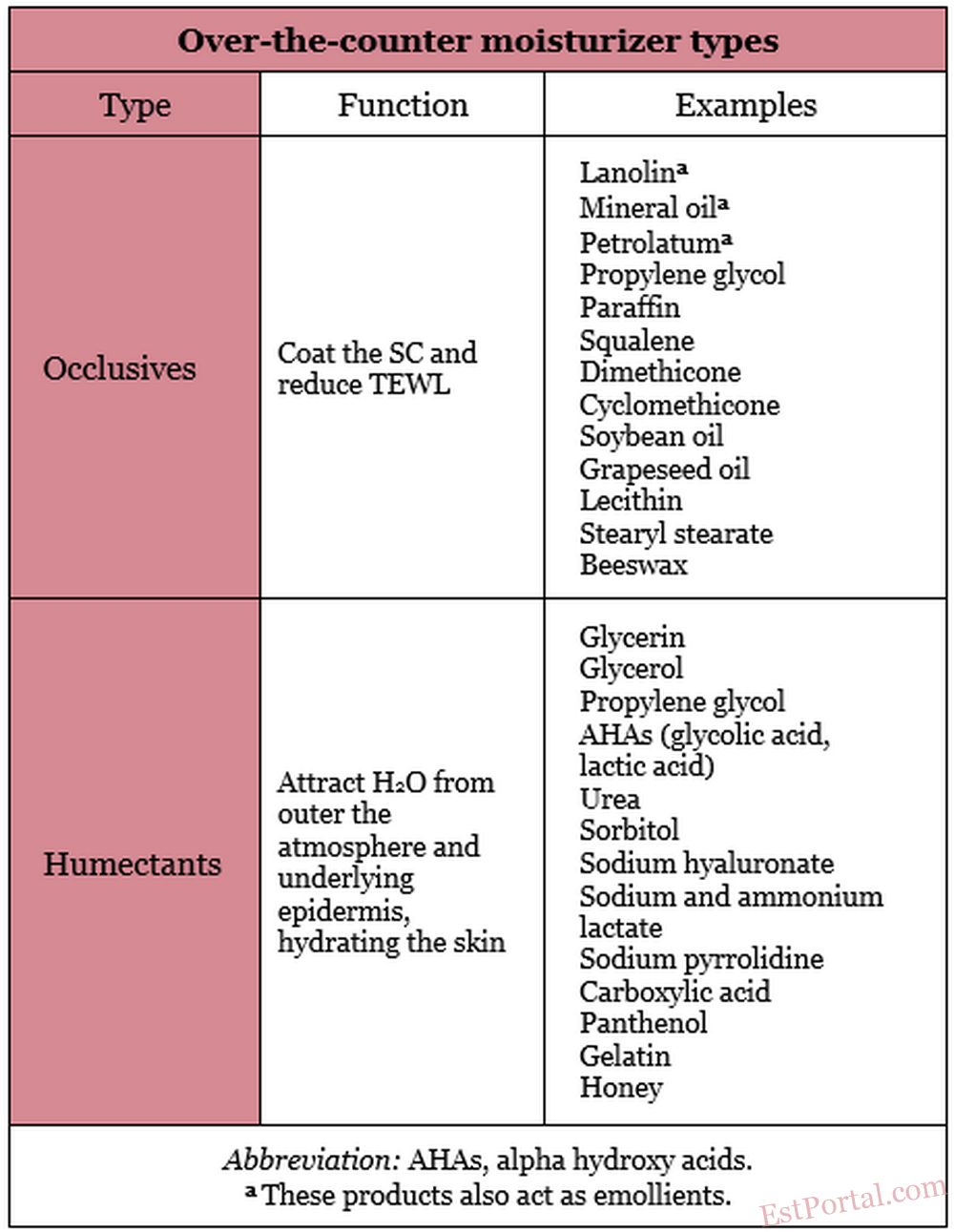
In addition to pharmacologic products beneficial in the treatment of xerosis and practical recommendations regarding what patients who have dry skin should avoid, there are several over-the-counter (OTC) moisturizers (eg, occlusives, humectants, and emollients) available that are effective in hydrating the skin (Table 2). Moisturizers are the third most often recommended type of OTC topical skin care product. [34] Awareness of the differences among moisturizer types is an important part of a practitioner’s knowledge base from which to suggest the most suitable products for a given patient’s skin type. Moisturizers are usually packaged as water-in-oil emulsions (eg, hand creams) and oil-in-water emulsions (eg, creams and lotions).
Table 2
Occlusives
When used in skin care products, occlusives, which are oily substances that can dissolve fats, coat the SC to inhibit TEWL. In addition to impeding TEWL, occlusives confer an emollient effect, and are therefore suitable products for treating xerosis. The most effective occlusive ingredients are petrolatum and mineral oil. Petrolatum, used as a skin care product since 1872, is considered one of the best moisturizers and a gold standard by which other occlusives are measured. [35] A resistance to water vapor loss that is 170 times that of olive oil is ascribed to petrolatum. [36] Unfortunately, petrolatum has such a greasy texture that some consumers find such products cosmetically unacceptable. Besides petrolatum and mineral oil, other frequently used occlusive ingredients include paraffin, squalene, silicone derivatives (dimethicone, cyclomethicone), soybean oil, grapeseed oil, propylene glycol, lanolin, lecithin, stearyl stearate, and beeswax. [37, 38] Derived from the sebaceous secretions of sheep, lanolin contains the important SC lipid cholesterol and can coexist with SC lipids as solids and liquids at physiologic temperatures. Lanolin has been deemed a sensitizer by some, although it has been demonstrated to be a weak allergen. [39] Lanolin may also be eschewed because it contains animal products. Although numerous moisturizers are now labeled ‘‘lanolin-free,’’ lanolin is still widely used. No occlusive ingredients provide long-lasting benefits. TEWL returns to its previous level once the occlusive agent is removed from the skin. Occlusives are typically used in combination with humectants because decreasing TEWL by more than 40% risks maceration, with elevated bacteria levels. [40]
Propylene glycol. An odorless liquid with antimicrobial and keratolytic properties, propylene glycol (PG) acts as an occlusive and a humectant. PG has been shown to facilitate the cellular penetration of some drugs, including steroids and minoxidil. PG is believed to be a weak sensitizer, but it may contribute to contact dermatitis by facilitating the penetration of allergens into the epidermis. [41]
Humectants
Humectants are water-soluble and hygroscopic substances. Humectants applied to the skin have the capacity to attract water from the external environment (in conditions with at least 80% humidity) and from the underlying skin layers. In low-humidity conditions, however, humectants may absorb water from the deeper epidermis and dermis, thus contributing to TEWL and aggravating skin dryness. [42] Consequently, humectants are more effective when combined with occlusive products. Several humectant products have also been identified as exhibiting emollient characteristics. [43] Humectants are incorporated into cosmetic moisturizers because they prevent product evaporation and thickening, which prolongs the product’s shelf-life. These products do not impart long-lasting antiwrinkle effects on the skin, however. Humectants, by drawing water into the skin, provoke a minor swelling of the SC, rendering a perception, which lasts for about 24 hours, of smoother skin with fewer wrinkles. Some humectants confer other benefits, such as bacteriostatic activity. [44] The most effective humectant ingredients in skin care products are glycerin and glycerol. Several other compounds function as active humectant ingredients, including alpha hydroxy acids, panthenol, carboxylic acid, sorbitol, sodium hyaluronate, sodium and ammonium lactate, sodium pyrrolidine, urea, propylene glycol, gelatin, honey, and other sugars. [38] Effective moisturizers usually include occlusive and humectant ingredients.
Glycerin. A strong humectant, glycerin exhibits hygroscopic activity comparable to that of NMF. [2] Investigators reported after a 5-year study comparing two high-glycerin moisturizers to 16 other popular moisturizers used by 394 patients who had severe xerosis that the high-glycerin products were the most effective, quickly restoring dry skin to normal hydration with longer-lasting results than the other moisturizers, which included petrolatum preparations. [45] In addition, glycerin has been shown, by way of ultrastructural analyses of skin treated with high-glycerin preparations, to expand the SC by enhancing corneocyte thickness and producing greater distance between layers of corneocytes. [46] Glycerin has also been demonstrated to stabilize and hydrate cell membranes and the enzymes required for desmosome degradation. [45]
Urea. Since the 1940s, urea has been included in many hand creams. [47] This dynamic compound is an end product of protein metabolism in mammals, the primary nitrogen-containing ingredient of urine, and an NMF constituent, and it displays humectant and mild antipruritic properties. [48] Combining urea with hydrocortisone, retinoic acid, [49, 50] and other ingredients has been shown to promote the cutaneous penetration by these agents. The Cosmetic Ingredient Review Expert Panel recently declared that urea does have the capacity to enhance the percutaneous absorption of other chemicals, and that urea is safe for use in cosmetic products. [51] There had been some earlier disagreement as to whether urea had exhibited such activity. A double-blind clinical study comparing 3% and 10% urea cream found that the study formulations were more effective in dry skin than the vehicle control. The 10% cream reduced TEWL but the 3% cream had no impact on TEWL, although the creams were reported to be equally effective. [52]
Hydroxy acids. Alpha hydroxy acids (AHAs) are a class of naturally occurring organic acids that have been found to function as humectants and exfoliants. The most frequently used AHAs in moisturizing formulations are glycolic and lactic acids (derived, respectively, from sugar cane and sour milk). Other AHAs include malic acid, citric acid, and tartaric acid. Glycolic and lactic acids were the first AHAs to become commercially available. It was shown more than 30 years ago that topical formulations containing AHAs exert significant effects on epidermal keratinization. [53] A decade ago, glycolic acid was demonstrated to exhibit photoprotective activity. [54] The only beta hydroxy acid (BHA), salicylic acid, which is derived from willow bark, wintergreen leaves, and sweet birch, acts as a chemical exfoliant and is included in synthetic form in various topical preparations. [55] AHAs and BHA erode corneocyte cohesiveness at the lowest levels of the SC, also influencing pH in the process, and break down desmosomes, thus facilitating desquamation. [56, 57]
Lactic acid. This prominent AHA is unusual in that itis also a component of NMF. Lactic acid was firstused in dermatologic therapy in 1943 to treat ichthyosis. [58] In vitro and in vivo experiments have since shown that lactic acid can enhance ceramide production by keratinocytes. [59, 60] In addition, a double-blind vehicle-controlled study using an 8% L-lactic acid formula revealed that the AHA was a superior treatment than the vehicle for photoaged skin, rendering statistically significant improvements in sallowness, skin coarseness, and blotchiness. [61]
Emollients
Included in cosmetics to hydrate, soften, and smooth the skin, emollients are composed mainly of lipids and oils. A smooth skin surface is rendered by these substances that act by filling in the gaps between desquamating corneocytes. [62] Emollient formulations enhance cohesion, yielding a flattening of the curled edges of individual corneocytes. [2] As a result, a smoother skin surface decreases friction while improving light refraction. There are several classes of emollients, including astringent, dry, fatting, and protective, along with protein rejuvenators. [38] There are also primarily occlusive ingredients that confer an emollient effect, such as lanolin, mineral oil, and petrolatum.
Moisturizers are generally regarded as safe, with reports of adverse effects exceedingly rare. Products containing preservatives, perfumes, solubilizers, sunscreens, and some other classes of compounds have been linked to reports of allergic contact dermatitis. Lanolin, propylene glycol, vitamin E, and Kathon CG have been associated with contact dermatitis. [63, 64]
Collagen and Polypeptide Ingredients
Most of the collagen ‘‘extracts’’ contained in many expensive moisturizers touted for replacing collagen lost with aging have a molecular weight of 15,000 to 50,000 daltons, but only compounds with a molecular weight of 5000 daltons or less can actually penetrate the SC. [40] Nevertheless, the collagen and other hydrolyzed proteins and polypeptides produce a temporary film on the epidermis that, once the product dries, fills in surface irregularities. A subtle stretching out of fine skin wrinkles is provided by the film created by these products. This fuller or slightly plumper appearance can be further enhanced with the addition of a humectant. Formulations with collagen and polypeptide ingredients confer little or no effect on TEWL, but are typically labeled as moisturizers and firming creams.
Skin sensitivity
Sensitive Versus Resistant
Resistant skin is characterized by a robust SC that strongly protects the skin from allergens and other exogenous environmental irritants. Erythema and acne are rare in people who have resistant skin. Erythema may arise if an individual is overexposed to the sun; acne may emerge because of stress or hormonal fluctuations. Individuals who have resistant skin can use most skin care products without fear of adverse reactions (eg, acne, rashes, or a stinging response). The same qualities that allow for such an advantage, however, also render several products ineffective in such individuals, who have an exceedingly high threshold for product ingredient penetration and bioefficacy. Consequently, people who have resistant skin may be unable to detect differences among cosmetic skin care formulations because most products are too weak to cross the potent SC to impart benefits.
Sensitive skin is a more complex phenomenon and more difficult to characterize. It is also becoming increasingly common. [65] Most patients who present to a dermatologist complaining of sensitive skin are healthy women of childbearing age. Fortunately, with age, the incidence of sensitive skin seems to decrease. As the prevalence of sensitive skin has increased, so too has the number of products marketed as suitable for the treatment of sensitive skin. There are variations in the qualities of sensitive skin. There are four discrete subtypes: acne type (propensity to develop acne, blackheads, or whiteheads), rosacea type (tendency toward recurrent flushing, facial redness, and experiencing hot sensations), stinging type (proclivity to experiencing stinging or burning sensations), and allergic type (prone to manifesting erythema, pruritus, and skin flaking). Each of these subtypes presents distinct treatment challenges to the practitioner because products designed and marketed for sensitive skin are not necessarily appropriate for all sensitive skin subtypes. Despite such differences, the four subtypes of sensitive skin share one significant feature: inflammation. One consistent focus in any sensitive skin treatment program therefore is decreasing and eliminating inflammation. For patients who present with more than one type of sensitive skin, the treatment is understandably more complex and challenging.
Acne Type
Although incidence and prevalence rates vary, acne is by far the most common skin disease, typically affecting adolescents and young adults, equally by gender, between the ages of 11 and 25 years. The second-largest demographic group that suffers from acne in appreciable numbers is adult women, who exhibit a hormonal component to their acne. The pathogenesis of this conspicuous and, therefore, stressful condition originates from the intersection of four main factors: increased sebum production; clogged pores due to dead keratinocytes inside the hair follicles adhering more strongly than in those without acne (higher sebum production may also promote such cellular clinging), the presence of the bacteria Propionibacterium acnes, and inflammation. Although acne can occur in various idiopathic presentations, the quintessential feature is the adherence of dead keratinocytes in the hair follicles as a result of increased sebum production, yielding clogged follicles and the emergence of a papule or pustule. Subsequently, P. acnes migrates into the hair follicle, intersecting with the collected sebum and dead keratinocytes. This interaction spurs the release of cytokines and other inflammatory factors that engender the inflammatory response leading to the formation of the characteristic redness and pus. High levels of primary cytokines, chemokines, and other inflammatory markers are usually present in chronic inflammatory skin conditions such as acne. [3]
The treatment of acne targets the four primary etiologic factors: reducing sebum production (with retinoids, oral contraceptives, or stress reduction), unclogging pores (with retinoids, AHAs, or BHA), eradicating bacteria (with benzoyl peroxide, sulfur, antibiotics, or azelaic acid), and decreasing inflammation.
Rosacea Type
According to the National Rosacea Society, 14 million Americans, [66] usually adults between 25 and 60 years of age, are affected by rosacea. This acneiform condition, the pathophysiology of which has yet to be completely elucidated, shares some symptoms with acne, specifically facial redness, flushing, and papules; however, rosacea is also characterized by the formation of prominent telangiectases, the primary manifestation of the condition. Topical rosacea treatments target the use of anti-inflammatory ingredients to decrease the dilation of the blood vessels and the avoidance of exposure to factors that trigger or aggravate symptoms. The goal of rosacea therapy is to reduce vascular reactivity, attack free radicals or reactive oxygen species (ROS), inhibit immune function, and interfere with eosinophilic activity, degranulation of mast cells (which often colocalize to areas of eosinophil-mediated disease), and the arachidonic acid pathway. Eosinophils are pleiotropic multifunctional leukocytes involved in initiating and promoting numerous inflammatory responses. [67, 68] The most effective anti-inflammatory ingredients (many of which are derived from botanical origins) in the myriad topical rosacea therapies include aloe vera, arnica, chamomile, colloidal oatmeal, cucumber extract, feverfew, licochalcone, niacinamide, Quadrinone, salicylic acid, sulfacetamide, sulfur, witch hazel, and zinc. [69] Various prescription anti-inflammatory products, including antibiotics, immune modulators, and steroids, are also available to treat rosacea.
Stinging Type
The stinging response is a nonallergic neural sensitivity that some people experience in reaction to various triggers. Several tests are available to identify ‘‘stingers’’ or the stinging tendency. The lactic acid stinging test is a particularly well-regarded method of evaluating individuals who report invisible and subjective cutaneous irritation. The stinging sensation is not necessarily linked to erythema, because many patients feel stinging without manifesting redness. [70] Rosacea patients exhibiting facial flushing are more susceptible to experiencing stinging caused by exposure to lactic acid. [71] Patients who are confirmed to have the stinging subtype of sensitive skin should avoid topical products containing the following ingredients: alpha hydroxy acids (particularly glycolic acid), benzoic acid, bronopol, cinnamic acid compounds, Dowicil 200, formaldehyde, lactic acid, propylene glycol, quaternary ammonium compounds, sodium lauryl sulfate, sorbic acid, urea, or vitamin C.
Allergic Type
A recent epidemiologic survey in the United Kingdom found that over 1 year 23% of women and 13.8% of men exhibited an adverse reaction to a personal care product (eg, deodorants and perfumes, skin care products, hair care products, and nail cosmetics). [72] Further, numerous studies have shown that approximately 10% of dermatologic patients who are patch tested for anywhere from 20 to 100 ingredients manifest allergic sensitivity to at least one ingredient common in cosmetic products. [70] The most common allergens are fragrances and preservatives and the preponderance of people who experience such reactions are women aged 20 to 60 years. [73] Greater susceptibility to allergic reactions is seen among those who are overexposed to skin care products and patients who have an impaired SC, as manifested by xerosis. [74]
Based on the principles of the BSTI, people who have oily, sensitive skin require oil control. Such an individual would also likely require an acne or rosacea treatment regimen. Those who have dry, sensitive skin require treatment to achieve skin barrier repair. People who have sensitive, wrinkled skin would benefit from treatments intended to reduce present wrinkles and prevent the formation of new ones. Those who have sensitive, pigmented skin typically seek the removal of the pigmentary lesion and treatment to prevent additional pigmentation.
Skin pigmentation
Pigmented Versus Nonpigmented
This skin parameter does not pertain to skin color, but to the propensity to develop undesired hyperpigmentations on the face, chest, or arms. Skin conditions or lesions that require excision or treatment beyond skin care (eg, congenital nevi, seborrheic keratoses) are not considered within the realm of typical pigmented skin in the BSTI framework. Pigmentary conditions or changes that can be ameliorated with skin care products and minor dermatologic procedures, such as melasma, solar lentigos, ephelides, and postinflammatory hyperpigmentation, do fall within this rubric, however. Some patients pay significant sums in the pursuit of satisfactory treatment of these anxiety-producing pigmentary problems; for practitioners to know how best to treat them, the origin of pigmentation should be clearly understood.
The skin pigment melanin is derived from the enzymatic breakdown of tyrosine by tyrosinase into dihydroxyphenylalanine and then dopaquinone, ultimately yielding the two melanin types, eumelanin and pheomelanin. [75] The more prevalent type, eumelanin, regularly correlates with the visual phenotype. [76] More melanin is produced in darker-skinned individuals than lighter-skinned ones. The larger melanosomes in darker-skinned people accommodate more melanin and therefore decompose more slowly than in lighter-skinned people. [77] Melanin is synthesized by melanocytes and then transferred by way of melanosomes to keratinocytes. Ultraviolet (UV) irradiation can also induce melanogenesis, however, which under these circumstances represents the skin’s defense to the insult of UV exposure. In this reaction to UV irradiation, melanocytes accelerate the production of melanin and its transfer to keratinoctyes, [78] resulting in the darkening of the skin in affected areas.
One melanocyte is usually linked to approximately 30 keratinocytes. In the process of transferring through melanosomes, the melanocyte loads the melanosome with melanin and then attaches to the keratinocytes. The keratinocytes surround the melanosome and absorb the melanin after the protease-activated receptor (PAR)-2 is activated. [79] PAR-2, which is expressed in keratinocytes but not melanocytes, is a seven transmembrane G-protein-coupled trypsin/tryptase receptor activated by a serine protease cleavage. It is believed that PAR-2 regulates pigmentation by way of exchanges between keratinocytes and melanocytes. [80]
The development of skin pigmentation can be inhibited by way of two main pathways: inhibiting tyrosinase, thereby preventing melanin formation, and impeding the transfer of melanin into keratinocytes. Effective tyrosinase inhibitors include hydroquinone, vitamin C, kojic acid, arbutin, mulberry extract, and licorice extract. Two proteins found in soy — soybean trypsin inhibitor (STI) and Bowman-Birk inhibitor (BBI) — have been identified as agents that have the capacity to impede the development of skin pigmentation. In addition to their depigmenting activity, STI and BBI have also been demonstrated to prevent UV-induced pigmentation in vitro and in vivo. [81] STI and BBI impart such effects by inhibiting the cleavage of PAR-2, and are therefore believed to affect melanosome transfer into keratinocytes. This pivotal transfer of melanosomes from melanocytes to keratinocytes has also been shown to be inhibited with the introduction of niacinamide, a derivative of vitamin B3. [82] As the most effective PAR-2 blockers, soy and niacinamide are the primary agents for impeding melanin transfer to keratinocytes.
Within the two approaches to hindering melanin formation, there are three types of topical agents useful in exerting such influence. Besides the tyrosinase inhibitors and PAR-2 blockers, exfoliating agents, such as AHAs, BHA, and retinoids, can accelerate cell turnover to such an extent that it outpaces melanin production. Procedures, such as microdermabrasion, and instruments, such as facial scrubs, can also be used for these purposes. Any skin care regimen focused on reducing or eliminating the development of unwanted pigmentation should also include the use of broad-spectrum sunscreens. Sun avoidance remains the most effective way to prevent pigmentary changes to the skin, among other deleterious effects. In the BSTI, an individual who has a tendency to form unwanted dyschromias would be considered to have type ‘‘P’’ skin and, otherwise, type ‘‘N’’ skin.
Skin aging
Wrinkled Versus Tight
Cutaneous aging is a dynamic, multifactorial process under endogenous and exogenous influences. The etiologic factors have traditionally been considered so distinct that two discrete processes have been described: natural intrinsic aging is genetically driven, or cellularly programmed, inevitable, and eventually results in visible skin alterations; extrinsic aging, which also manifests in cutaneous changes, results from the chronic exposure to various environmental insults and is therefore avoidable. Recent insights suggest that the primary factor implicated in extrinsic aging — UV radiation — may actually alter the normal course of natural aging. If this is the case, intrinsic and extrinsic aging are less distinct than previously believed.
This brief discussion considers these processes separately. In recent years, the function of telomeres, the specialized structures that protect the ends of chromosomes, has come to be identified as one of the keys to intrinsic aging. Telomere length is known to diminish with age, and this erosion is seen as tantamount to a gauge by which to measure chronologic aging. This veritable internal aging clock mechanism is the basis for one of the currently favored theories on aging. [83] The enzyme telomerase, which stabilizes or lengthens telomeres, is expressed in about 90% of all tumors but does not appear in many somatic tissues. [83] This phenomenon implies that most cancer cells, unlike healthy cells, are not programmed for apoptosis, or cell death, essentially placing aging and cancer on opposite sides of the same coin. The epidermis is one of the few regenerative tissues to express telomerase. [84] Currently, no treatment options target telomerase because current data are insufficient regarding the safety of extending telomere length.
Extrinsic aging, as implied in the definition, is preventable and is thus subject to human control. Individuals can make a concerted effort to limit exposure to the primary causes of exogenous aging. These etiologic factors include smoking, other pollution, poor nutrition, excessive alcohol consumption, and especially solar exposure. Cutaneous damage results from exposure to UV irradiation through various mechanisms, including the formation of sunburn cells by way of pyrimidine and thymine dimers, collagenase synthesis, and the promotion of an inflammatory response. Significantly, signaling through the p53 pathway after telomere disruption induced by UV irradiation (UVB in particular) has been linked to aging and photodamage. [85, 86] Photoaging, photocarcinogenesis, and photoimmunosuppression are well known adverse effects of UV (particularly UVA), although much more remains to be learned about the mechanisms through which UV irradiation fosters harmful effects. [87] Because UV irradiation inhibits DNA and accelerates telomere shortening, this primary source of extrinsic aging can be considered to influence the course of intrinsic aging.
Rhytid formation, which begins in the lower dermal layers of the skin, is the quintessential manifestation of aging skin. Few skin care product formulations can actually penetrate far enough into the dermis to alter or reverse deep wrinkles, despite the wealth of products advertising otherwise and the significant outlay of consumers’ money for such products. Antiaging skin care consequently focuses on the prevention of wrinkle formation. [88] Because it is well known that the three main structural components of the skin — collagen, elastin, and HA — decline with age, the primary goal in product formulation is to prevent the degradation of one or more of these key constituents. Although there are no topical products that can deliver these substances deeply into the epidermis, despite what the marketing might indicate, some products do promote the natural production of these important compounds. Topically, retinoids, vitamin C, and copper peptide have been demonstrated to stimulate collagen production, [89, 90] and oral vitamin C is also believed to have the same capacity. [91] In addition, retinoids have been demonstrated in animal models to promote the synthesis of HA and elastin, [92, 93] whereas glucosamine supplementation is also believed to augment HA levels. [94] As of yet, no products have been shown or approved for the stimulation of elastin production.
Another important target of wrinkle prevention that occurs beneath the skin is reducing inflammation, because inflammation is known to contribute to collagen, elastin, and HA degradation. Antioxidants play a significant role in this approach because they protect the skin by way of several mechanisms that are becoming better understood and elucidated. For example, ROS acting directly on growth factor and cytokine receptors in keratinocytes and dermal cells can engender skin inflammation. Nevertheless, much remains to be learned about the direct roles of growth factors and cytokines in cutaneous aging. Currently, growth factors and cytokines are known to function synergistically in a complex mechanism involving various types of growth factors and cytokines. [95] It is believed that UV irradiation triggers a cascade of events, acting on growth factor and cytokine receptors in keratinocytes and dermal cells, leading to downstream signal transduction by activating mitogen-activated protein (MAP) kinase pathways (extracellular signal-regulated kinase, c-jun N-terminal protein kinase, and p38). These then collect in cell nuclei, forming cFos/cJun complexes of transcription factor activator protein 1, and inducing the matrix metalloproteinases collagenase, 92 kDa gelatinase, and stromelysin to break down collagen and other cutaneous connective tissue. [96, 97]
The direct effects of ROS on the aging process and skin aging are more clearly understood. Kang and colleagues have shown that free radical activation of the MAP kinase pathways induces collagenase synthesis, which leads to the breakdown of collagen. [97] Inhibiting these pathways by the use of antioxidants is believed to deter photoaging by preventing collagenase synthesis and its ensuing harmful effects on collagen. In experiments using human skin, Kang and colleagues found that the pretreatment of skin with the antioxidants genistein and N-acetyl cysteine inhibited the UV induction of the cJun-driven enzyme collagenase.
A plethora of antioxidants are used as ingredients in topical skin care products, including vitamins C and E, coenzyme Q10, and those derived from botanical sources, such as caffeine, coffee berry, ferulic acid, feverfew, grape seed extract, green tea, idebenone, mushrooms, polypodium leucotomos, pomegranate, Pycnogenol, reseveratrol, rosemary, and silymarin. Although copious evidence is presented in the literature identifying the antioxidant potency of these ingredients, their efficacy in topical formulations intended to combat the cutaneous signs of aging has not yet been established. It likely that in the not-too-distant future technological innovation in tissue engineering and gene therapy will yield breakthroughs in the therapeutic uses of growth factors, cytokines, and telomerase. [98] It is equally probable that some such applications will be included in the dermatologic armamentarium. In the interim, several practical steps can be taken to mitigate or even prevent extrinsic skin aging, including: avoiding/limiting exposure to the sun (particularly from 10 AM to 4 PM), using broad-spectrum sunscreen when avoiding the sun is impossible, avoiding cigarette smoke and pollution, taking oral antioxidant supplements or topical antioxidant formulations, regularly using prescription retinoids, and eating a diet high in fruits and vegetables. Protecting the skin is a key step in fundamental skin care (Box 2).
Box 2
Four elements of fundamental skin care
- Mild cleansing.
- Hydrating. Effective moisturization (with humectants and emollients).
- Replenishing. With lipids, ceramides and fatty acids.
- Protecting: UV protection, increased humidity.
Skin type combinations and changes
Because the skin parameters together describe the simultaneous state or tendencies of the skin along four different spectra, the permutations of the four skin parameters yield 16 different skin types. The BSTI skin typing system can assist individuals, once they have identified their skin type, in gaining insight into treating their particular skin problem areas and provide guidance as to the most suitable OTC products for their skin. For example, an individual who has oily, sensitive, nonpigmented, wrinkled skin (the OSNW skin type) would be best served by using products with retinoids and antioxidants. A person who has dry, sensitive, nonpigmented, tight skin (the DSNT skin type), would be advised to use products with ingredients intended for skin barrier repair. Although the BSTI can provide significant guidance for one’s skin care choices, an individual’s skin type can change, especially because of stress and exposure to variable environments (eg, when traveling to a region with a different climate). This phenomenon should be considered by patients and physicians in arriving at an overall skin type assessment. In addition, particular skin features, proclivities, or manifestations are seen in certain skin types, which is important to acknowledge when using skin care products based on the BSTI skin typing system. For instance, pigmented, wrinkled skin (PW) is more typical in an individual who has a significant history of sun exposure, resulting in wrinkles and solar lentigos. Dark skin is more common in individuals characterized as PT types; light skin is a common feature of those described as NW types. As for certain cutaneous conditions, rosacea is observed in OSNW skin types more often than in those who have other skin types. Eczema is more typical in people who have the DS combination than in individuals who have other skin types. Acne is associated with OS skin more than any other skin type.
Summary
The categories used to describe skin types have changed little over the last century, whereas the skin care product market has undergone rapid innovation and exponential growth. The four traditional labels used to depict skin type cannot adequately characterize the actual variations observed in skin type nor provide sufficient guidance for the proper selection of skin care products. There are four basic dichotomies or parameters that more accurately characterize skin types and these have only recently been introduced. By evaluating skin according to these parameters — dry or oily, sensitive or resistant, pigmented or nonpigmented, and wrinkled or unwrinkled — and thus differentiating among the 16 permutations of possible skin types, consumers can more easily identify the most suitable topical treatments for their skin. An individual’s BSTI four-letter descriptive skin type is derived from answers to a 64-item self-administered questionnaire. The BSTI is based on the understanding that the various parameters are not mutually exclusive; an individual’s skin should be described along all four spectra simultaneously. Once armed with a patient’s BSTI score, physicians are equipped with significant information that can assist them in treating numerous skin conditions and confidently recommending the most appropriate OTC topical skin care products for their patients. Myriad topical skin care products are available that can meet the needs of most of the 16 skin types.
References
- Baumann L. The skin type solution. New York: Bantam Dell; 2006.
- Chernosky M. E. Clinical aspects of dry skin. J Soc Cosmet Chem 1976;27:365–76.
- Elias P. M. Stratum corneum defensive functions: an integrated view. J Invest Dermatol 2005;125(2):183–200.
- Wildnauer R. H, Bothwell JW, Douglass AB. Stratum corneum biomechanical properties. I. Influence of relative humidity on normal and extracted human stratum corneum. J Invest Dermatol 1971;56(1):72–8.
- Orth D., Appa Y. Glycerine: a natural ingredient for moisturizing skin. In: Loden M, Maibach H, editors. Dry skin and moisturizers. Boca Raton (FL): CRC Press; 2000. p. 214.
- Rawlings A., Hope J., Rogers J., et al. Skin dryness — what is it? [abstract]. J Invest Dermatol 1993;100:510.
- Ekholm I. E., Brattsand M., Egelrud T. Stratum corneum tryptic enzyme in normal epidermis: a missing link in the desquamation process? J Invest Dermatol 2000;114(1):56–63.
- Elias P. M. The epidermal permeability barrier: from the early days at Harvard to emerging concepts. J Invest Dermatol 2004;122(2):XXXVI–IX.
- Scott I. R., Harding C. R. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol 1986;115(1):84–92.
- Sato J., Denda M., Chang S., et al. Abrupt decreases in environmental humidity induce abnormalities in permeability barrier homeostasis. J Invest Dermatol 2002;119(4):900–4.
- Wang F., Feng X. C., Li Y. M., et al. Aquaporins as potential drug targets. Acta Pharmacol Sin 2006;27(4):395–401.
- Sougrat R., Morand M., Gondran C., et al. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol 2002;118(4):678–85.
- Takenouchi M., Suzuki H., Tagami H. Hydration characteristics of pathologic stratum corneum — evaluation of bound water. J Invest Dermatol 1986;87(5):574–6.
- Warner R. R., Bush R. D., Ruebusch N. A. Corneocytes undergo systematic changes in element concentrations across the human inner stratum corneum. J Invest Dermatol 1995;104(4):530–6.
- Warner R. R., Myers M. C., Taylor D. A. Electron probe analysis of human skin: element concentration profiles. J Invest Dermatol 1988;90(1):78–85.
- Warner R. R., Myers M. C., Taylor D. A. Electron probe analysis of human skin: determination of the water concentration profile. J Invest Dermatol 1988;90(2):218–24.
- Ma T., Hara M., Sougrat R., et al. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem 2002;277(19):17147–53.
- Yang B., Verkman A. S. Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem 1997;272(26):16140–6.
- Dumas M., Gondran C., Barre P., et al. Effect of an Ajuga turkestanica extract on aquaporin 3 expression, water flux, differentiation and barrier parameters of the human epidermis. Eur J Dermatol 2002;12(6):XXV–VI.
- Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol 2004;123(1):1–12.
- Clarys P., Barel A. Quantitative evaluation of skin surface lipids. Clin Dermatol 1995;13(4):307–21.
- Thody A.J., Shuster S. Control and function of sebaceous glands. Physiol Rev 1989;69(2):383–416.
- Elias P. M., Fritsch P. O., Lampe M., et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest 1981;44(6):531–40.
- Gomez E. C. Differential effect of 13-cis-retinoic acid and an aromatic retinoid (Ro 10–9359) on the sebaceous glands of the hamster flank organ. J Invest Dermatol 1981;76(1):68–9.
- Geiger J. M. Retinoids and sebaceous gland activity. Dermatology 1995;191(4):305–10.
- Mathers W. D., Lane J. A. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol 1998;438:349–60.
- Tiffany J. M. The role of meibomian secretionin the tears. Trans Ophthalmol Soc U K 1985;104(Pt 4):396–401.
- Fluhr J. W., Mao-Qiang M., Brown B. E., et al. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol 2003;120(5):728–37.
- Fluhr J. W., Gloor M., Lehmann L., et al. Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol 1999;79(6):418–21.
- Pochi P. E., Strauss J. S., Downing D. T. Age-related changes in sebaceous gland activity. J Invest Dermatol 1979;73(1):108–11.
- Walton S., Wyatt E. H., Cunliffe W. J. Genetic control of sebum excretion and acne — a twin study. Br J Dermatol 1988;118(3):393–6.
- De Pedrini P., Rapisarda R., Spano G. The effect of ketoconazole on sebum secretion in patients suffering from acne and seborrhoea. Int J Tissue React 1988;10(2):111–3.
- Goldstein J. A., Socha-Szott A., Thomsen R. J., et al. Comparative effect of isotretinoin and etretinate on acne and sebaceous gland secretion. J Am Acad Dermatol 1982;6(Suppl 4 Pt 2):760–5.
- Vogel C. A., Balkrishnan R., Fleischer A. B., et al. Over-the-counter topical skin care products — a common component of skin disease management. Cutis 2004;74(1):55–67.
- Morrison D. Petrolatum. In: Loden M., Maibach H., editors. Dry skin and moisturizers. Boca Raton (FL): CRC Press; 2000. p. 251.
- Spruit D. The interference of some substances with the water vapour loss of human skin. Dermatologica 1971;142(2):89–92.
- Draelos Z. Moisturizers. In: Draelos Z., editor. Atlas of cosmetic dermatology. New York: Churchill Living- stone; 2000. p. 83.
- Kraft J. N., Lynde C. W. Moisturizers: what they are and a practical approach to product selection. Skin Therapy Lett 2005;10(5):1–8.
- Kligman A. M. The myth of lanolin allergy. Contact Dermatitis 1998;39:103–7.
- Wehr R. F., Krochmal L. Considerations in selecting a moisturizer. Cutis 1987;39(6):512–5.
- Hannuksela M. Glycols. In: Loden M., Maibach H., editors. Dry skin and moisturizers. Boca Raton (FL): CRC Press; 2000. p. 413–5.
- Idson B. Dry skin: moisturizing and emolliency. Cosmetics & Toiletries 1992;107:69–78.
- Del Rosso J. Q. Cosmeceutical moisturizers. In: Draelos Z. D., editor. Procedures in cosmetic dermatology series: cosmeceuticals. 1st edition. Philadelphia: Elsevier; 2005. p. 97–102.
- Mitsui T., editor. New cosmetic science. New York: Elsevier; 1997. p. 134.
- Orth D., Appa Y. Glycerine: a natural ingredient for moisturizing skin. In: Loden M, Maibach H, editors. Dry skin and moisturizers. Boca Raton (FL): CRC Press; 2000. p. 217.
- Orth D., Appa Y., Contard E., et al. Effect of high glycerin therapeutic moisturizers on the ultrastructure of the stratum corneum. Poster presentation at the 53rd annual meeting of the American Academy of Dermatology. New Orleans (LA), February 1995.
- Harding C., Bartolone J., Rawlings A. Effects of natural moisturizing factor and lactic acid isomers on skin function. In: Loden M., Maibach H., editors. Dry skin and moisturizers. Boca Raton (FL): CRC Press; 2000. p. 229.
- Kligman A. M. Dermatologic uses of urea. Acta Derm Venereol 1957;37:155–9.
- Wohlrab W. The influence of urea on the penetration kinetics of topically applied corticosteroids. Acta Derm Venereol 1984;64:233–8.
- Wohlrab W. Effect of urea on the penetration kinetics of vitamin A acid in human skin. Z Hautkr 1990;65:803–5.
- The cosmetic ingredient review (CIR) expert panel. Final report of the safety assessment of urea. Int J Toxicol 2005;24(Suppl 3):1–56.
- Serup J. A double-blind comparison of two creams containing urea as the active ingredient. Assessment of efficacy and side-effects by non-invasive techniques and a clinical scoring scheme. Acta Derm Venereol Suppl (Stockh) 1992;177:34–43.
- Van Scott E. J., Yu R. J. Control of keratinization with alpha hydroxy acids and related compounds. I. Topical treatment of ichthyotic disorders. Arch Dermatol 1974;110:586–90.
- Perricone N. V., Dinardo J. C. Photoprotective and antiinflammatory effects of topical glycolic acid. Dermatol Surg 1996;22:435–7.
- Draelos Z. D. Rediscovering the cutaneous benefits of salicylic acid. Cosmetic Dermatology 1997;10(Suppl 4):4–5.
- Van Scott E. J., Yu R. Hyperkeratinization, corneocyte cohesion, and alpha hydroxy acids. J Am Acad Dermatol 1984;11:867–79.
- Berardesca E., Distante F., Vignoli G. P., et al. Alpha hydroxyacids modulate stratum corneum barrier function. Br J Dermatol 1997;137:934–8.
- Stern E. Topical application of lactic acid in the treatment and prevention of certain disorders of the skin. Urol Cutaneous Rev 1943;50:106–7.
- Rawlings A. V., Davies V., Carlomusto M., et al. Effect of lactic acid isomers on keratinocyte ceramide synthesis, stratum corneum lipid levels and stratum corneum barrier function. Arch Dermatol Res 1996; 288:383–90.
- Harding C. R., Bartolone J., Rawlings A. V. Effectof natural mosturizing factor and lactic acid isomers on skin function. In: Loden M., Maibach H., editors. Dry skin and mosturizers. Boca Raton (FL): CRC Press; 2000. p. 236.
- Stiller M. J., Bartolone J., Stern R., et al. Topical 8% glycolic acid and 8% L-lactic acid creams for the treatment of photodamaged skin. A double-blind vehicle-controlled clinical trial. Arch Dermatol 1996;132:631–6.
- Draelos Z. Moisturizers. In: Draelos Z., editor. Atlas of cosmetic dermatology. New York: Churchill Livingstone; 2000. p. 85.
- Gonzalo M. A., de Argila D., Garcia J. M., et al. Allergic contact dermatitis to propylene glycol. Allergy 1999;54:82–3.
- Baumann L. S., Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg 1999;25:311–5.
- Draelos Z. D. Cosmetic selection in the sensitive-skin patient. Dermatol Ther 2001;14:194–9.
- National rosacea society website. Available at: http://www.rosacea.org/index.php. Accessed October 12, 2007.
- Rothenberg M. E., Hogan S. P. The eosinophil. Annu Rev Immunol 2006;24:147–74.
- Shakoory B., Fitzgerald S. M., Lee S. A., et al. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res 2004;24(5):271–81.
- Brown D. J., Dattner A. M. Phytotherapeutic approaches to common dermatologic conditions. Arch Dermatol. 1998;134(11):1401–4.
- Basketter D. A., Griffiths H. A. A study of the relationship between susceptibility to skin stinging and skin irritation. Contact Dermatitis. 1993;29(4):185–8.
- Lonne-Rahm S. B., Fischer T., Berg M. Stinging and rosacea. Acta Derm Venereol. 1999;79(6):460–1.
- Orton D. I., Wilkinson J. D. Cosmetic allergy: incidence, diagnosis, and management. Am J Clin Dermatol 2004;5(5):327–37.
- Mehta S. S., Reddy B. S. Cosmetic dermatitis current perspectives. Int J Dermatol 2003;42(7):533–42.
- Jovanovic M., Poljacki M., Duran V., et al. Contact allergy to Compositae plants in patients with atopic dermatitis. Med Pregl 2004;57(5–6):209–18.
- Freedberg I. M., Eisen A. Z., Wolff K., et al, editors. Fitzpatrick’s dermatology in general medicine. 5th edition. New York: McGraw-Hill; 1999. p. 996.
- Wakamatsu K., Kavanagh R., Kadekaro A. L., et al. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res 2006;19(2):154–62.
- Szabo G., Gerald A. B., Pathak M. A., et al. Racial differences in the fate of melanosomes in human epidermis. Nature 1969;222(198):1081–2.
- Hermanns J. F., Petit L., Martalo O., et al. Unraveling the patterns of subclinical pheomelanin-enriched facial hyperpigmentation: effect of depigmenting agents. Dermatology 2000;201(2):118–22.
- Briganti S., Ottaviani M., Picardo M. Chemical, pharmologic, and physical agents causing hypomelanosis. In: Nordlund JJ, editor. The pigmentary system. New York: Oxford University Press; 2006. p. 678.
- Seiberg M., Paine C., Sharlow E., et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol 2000;115(2):162–7.
- Paine C., Sharlow E., Liebel F., et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol 2001;116(4):587–95.
- Hakozaki T., Minwalla L., Zhuang J., et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol 2002;147(1):20–31.
- Boukamp P. Ageing mechanisms: the role of telomere loss. Clin Exp Dermatol 2001;26(7):562–5.
- Boukamp P. Skin aging: a role for telomerase and telomere dynamics? Curr Mol Med 2005;5(2):171–7.
- Kosmadaki M. G., Gilchrest B. A. The role of telomeres in skin aging/photoaging. Micron 2004;35(3):155–9.
- Kappes U. P., Luo D., Potter M., et al. Short– and longwave UV light (UVB and UVA) induce similar mutations in human skin cells. J Invest Dermatol 2006; 126(3):667–75.
- Marrot L., Beladi J. P., Meunier J. R. Importance of UVA photoprotection as shown by genotoxic related endpoints: DNA damage and p53 status. Mutat Res 2005;571(1-2):175–84.
- Baumann L. How to prevent photoaging? J Invest Dermatol. 2005;125(4):XII–XIII.
- Varani J., Warner R. L., Gharaee-Kermani M., et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000;114(3):480–6.
- Nusgens B. V., Humbert P., Rougier A., et al. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J Invest Dermatol 2001;116(6):853–9.
- Kockaert M., Neumann M. Systemic and topical drugs for aging skin. J Drugs Dermatol. 2003;2(4):435–41.
- Margelin D., Medaisko C., Lombard D., et al. Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. J Invest Dermatol 1996; 106(3):505–9.
- Tajima S., Hayashi A., Suzuki T. Elastin expression is up-regulated by retinoic acid but not by retinol in chick embryonic skin fibroblasts. J Dermatol Sci 1997;15(3):166–72.
- Matheson A. J., Perry C. M. Glucosamine: a review of its use in the management of osteoarthritis. Drugs Aging 2003;20(14):1041–60.
- Fitzpatrick R. E. Endogenous growth factors as cosmeceuticals. Dermatol Surg 2005;31(7 Pt 2):827–31 [discussion: 831].
- Fisher G. J., Voorhees J. J. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc 1998;3(1):61–8.
- Kang S., Chung J. H , Lee J. H , et al. Topical N-acetyl cysteine and genistein prevent ultraviolet-lightinduced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol 2003; 120(5):835–41.
- Ostler E. L , Wallis C. V., Aboalchamat B., et al. Telomerase and the cellular lifespan: implications of the aging process. J Pediatr Endocrinol Metab 2000; 13(Suppl 6):1467–76.
Leslie S BAUMANN – MD, Baumann Cosmetic and Research Institute
Reprinted from ResearchGate