Розацеа: оновлення медикаментозної терапії
Розацеа є поширеним хронічним дерматозом, який переважно уражає центральну частину обличчя, включаючи щоки, ніс, очі, підборіддя і лоб.1 Первинні шкірні прояви включають чутливу шкіру, стійку еритему, папули, пустули та телеангіектазії. Хоча інтенсивність симптомів може тимчасово зменшуватися, у довгостроковій перспективі дерматоз у більшості пацієнтів має тенденцію до повільного прогресуючого перебігу.2
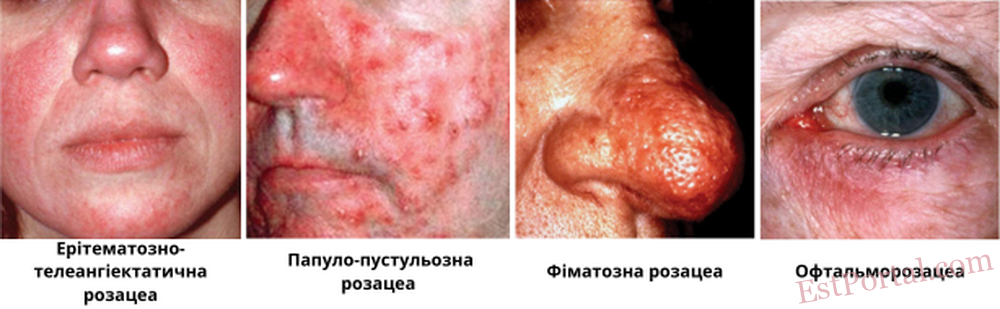
Національний комітет з питань розацеа поділяє розацеа на чотири основні підтипи:
- еритематозно-телеангіектазічний;
- папульозно-пустульозний;
- фіматозний;
- офтальмологічний.3
Можлива трансформація одного підтипу в інший.4 Правильне визначення підтипів може допомогти у виборі терапевтичної стратегії.
Розацеа уражає до 10% загальної чисельності населення і, як правило, дебютує у віці 30-50 років.5 Дерматоз особливо поширений серед людей зі світлою шкірою північного європейського походження.6 Частіше зустрічається у жінок,5 проте чоловіки більш схильні до розвитку інфільтрованих і фіматозних змін шкіри, особливо в області носа.
Патофізіологія є багатофакторною і наразі до кінця не вивчена. Було запропоновано багато факторів, що претендують на етіологічну роль, зокрема судинні аномалії, шлунково-кишкові розлади, дегенерація матриксу, аномалії сальних залоз, мікробна активність і зміни вродженого імунного захисту.8,9
Розацеа може викликати психологічні розлади, такі як збентеження, тривожність і низьку самооцінку, що негативно впливають на якість життя. Ці негативні наслідки мають бути враховані при лікуванні хворих на розацеа.10,11 Консервативні заходи, такі як запобігання загостренням, належний догляд за шкірою, а також використання маскуючої косметики та фотозахисту, також мають бути включені до плану лікування.12
Звичайні методи лікування
Топічний метронідазол
Метронідазол уперше був застосований як ефективний засіб при розацеа у 1980 році.13 Хоча метронідазол є антибактеріальним та антипаразитарним агентом, його терапевтичні переваги при розацеа здебільшого пов’язані з протизапальними та антиоксидантними властивостями.14 Декілька досліджень показали, що топічний метронідазол значно зменшує кількість запальних уражень і почервоніння у порівнянні з плацебо, загалом добре переноситься, має низький рівень побічних ефектів і є ефективним у підтримці ремісії.15-18
Важливо відзначити, що різні композиції метронідазолу, незалежно від форми випуску (крем, гель або лосьйон) або концентрації (0,75% чи 1%),19-21 мають подібну ефективність. Використання метронідазолу один раз на добу виявилося таким самим ефективним, як і дворазове застосування.19,22 Крім того, у поєднанні із сонцезахисним кремом SPF 15 метронідазол може зменшувати розвиток телеангіектазій на обличчі.23 Слід зазначити, що топічний метронідазол класифікується як препарат категорії B при використанні у вагітних.
Топічна азелаїнова кислота
Азелаїнова кислота — є природною дикарбоновою кислотою, схваленою протягом останнього десятиліття для лікування легкої та помірної розацеа.24 Вона переважно застосовується у вигляді 15% гелю або 20% крему та чинить лікувальний ефект завдяки своїм протизапальним, антикератотичним і антибактеріальним властивостям.24 Декілька досліджень продемонстрували, що азелаїнова кислота є ефективнішою за плацебо у зменшенні кількості запальних елементів і ступеня еритеми.25-27
Об’єднані показники пацієнтів, які зазнали значного покращення при лікуванні азелаїновою кислотою, становили 70-80% у порівнянні з 50-55% у групі плацебо.28 Азелаїнова кислота також має відносно низьку частоту побічних ефектів, серед яких найчастіше спостерігалося печіння, поколювання і подразнення.26 Частота побічних ефектів вища у азелаїнової кислоти порівняно з метронідазолом, проте ці ефекти зазвичай є легкими і короткочасними.29
Попри те, що стандартний режим застосування азелаїнової кислоти передбачає нанесення двічі на день, було встановлено, що її застосування один раз на день є не менш ефективним.30 Потрібні додаткові дослідження для оцінки використання азелаїнової кислоти як підтримувальної терапії.28 При використанні у вагітних азелаїнова кислота класифікується як препарат категорії B.
Тетрацикліни
Застосування пероральних антибіотиків поза прямим призначенням для лікування розацеа було визнано ефективним понад 50 років тому. Терапевтичні переваги тетрациклінів при розацеа пов’язані головним чином з їх протизапальним, а не антибактеріальним механізмом, оскільки немає достатніх доказів ролі бактеріальної інфекції в патогенезі цього захворювання.31 Антибіотики тетрациклінового ряду особливо показані при офтальморозацеа, яка зазвичай зустрічається у більш ніж 50% пацієнтів із розацеа.
Тетрацикліни, які протипоказані вагітним жінкам, є найбільш часто використовуваними антибіотиками для лікування запальних папул і пустул.
Тетрацикліни другого покоління, включаючи міноциклін і, зокрема, доксициклін, є особливо безпечними та ефективними при пероральному лікуванні розацеа. На відміну від тетрацикліну, вони мають більшу біодоступність,32 швидший початок дії та можуть прийматися разом із їжею, що мінімізує шлунково-кишкові побічні ефекти. Крім того, тетрацикліни другого покоління потребують лише одноразового прийому на добу, що може покращити дотримання режиму лікування пацієнтами. Найважливішим є те, що вони ефективні при субантибактеріальній дозі, що дозволяє уникнути негативного впливу на ендогенну мікрофлору та, що найголовніше, поширення резистентності до антибіотиків.33
У нещодавніх клінічних випробуваннях33 було показано, що субантибактеріальні дози доксицикліну в добовій дозі 40 мг у пацієнтів із помірною та тяжкою розацеа значно зменшували кількість запальних папул і пустул порівняно з плацебо після 16 тижнів лікування, при цьому значне покращення спостерігалося вже на третьому тижні. Частота побічних ефектів була низькою і лише трохи вищою, ніж у групі плацебо. Найбільш поширеними побічними ефектами були назофарингіт (4,8%), діарея (4,4%) та головний біль (4,4%). При цьому не було зафіксовано жодного випадку фотосенсибілізації чи вагінального кандидозу.
Окреме дослідження показало, що ефективність лікування розацеа 40 мг доксицикліну порівнянна з 100 мг доксицикліну.34 Субантибактеріальна доза 40 мг доксицикліну для лікування розацеа була схвалена у США та Канаді. Водночас міноциклін не отримав схвалення, оскільки, порівняно з доксицикліном, спричиняє у п’ять разів більше побічних ефектів, серед яких гіперпігментація, гепатотоксичність і спричинений ним вовчак.35
Потрібні подальші дослідження щодо ефективності комбінованої терапії субантибактеріальними дозами доксицикліну та топічного метронідазолу, яка показала швидший початок дії та швидшу регресію запальних висипань порівняно з монотерапією метронідазолом.36
Нові та нещодавно з’явлені методи терапії
Топічний івермектин
Декілька топічних акарицидних засобів (перметрин 5%, кротамітон 10% та івермектин 1%) були вивчені як препарати для лікування розацеа. Всі вони в першу чергу впливають на кліщів Demodex folliculorum і Demodex brevis. Потенційна етіологічна роль цих кліщів у розвитку розацеа обговорюється вже багато років.9 Останнім часом інтерес до кліщів відновився у зв’язку з дослідженнями, які показали, що антигенні білки, які виробляє симбіотична для кліща бактерія Bacillus oleronius, можуть посилювати запальні реакції при папульозно-пустульозній, еритематозно-телеангіектазічній38 і очній формах розацеа.37 Цей патогенний сценарій, у якому ключову роль відіграють бактерії Bacillus oleronius, а не самі кліщі, пояснює ефект антибактеріальних препаратів при розацеа.
Було опубліковано численні повідомлення39-41 про випадки успішного лікування розацеа топічними акарицидними препаратами, проте контрольованих рандомізованих досліджень поки що недостатньо. Наразі проводиться третя фаза рандомізованих клінічних досліджень, які вивчають ефективність і безпеку 1% крему івермектину в порівнянні з ефективністю і безпекою 0,75% крему метронідазолу та 15% гелю азелаїнової кислоти при розацеа.42-44 Очікується, що результати будуть доступні найближчим часом.
Топічний брімонідин і оксиметазолін
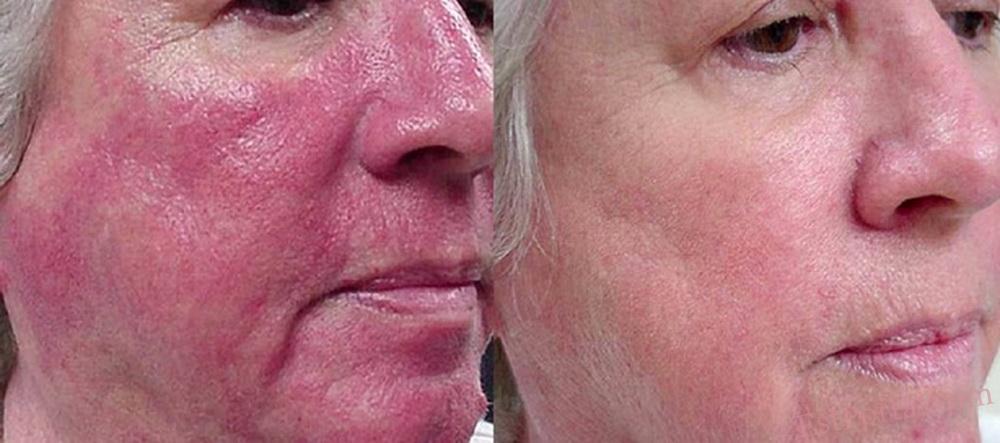
Дифузна і стійка еритема обличчя давно є клінічною проблемою у терапії розацеа.5 Одним із чинників, що спричиняє еритему, є аномальні вазомоторні реакції шкіри, які призводять до розширення поверхневих кровоносних судин обличчя.45 Реакція судин на вазоактивні стимули викликала інтерес до вивчення агоністів α2-адренорецепторів як терапевтичних засобів, що усувають еритему.45
Брімонідин тартрат 0,33% гель, схвалений FDA США у серпні 2013 року та Міністерством охорони здоров’я Канади у лютому 2014 року, є останнім доповненням до арсеналу лікування розацеа і першим топічним препаратом, схваленим для лікування еритеми обличчя при розацеа. Брімонідин (спочатку доступний у формі очних крапель для лікування глаукоми) є високо селективним агоністом α2-адренорецепторів з потужною судинозвужувальною активністю.46
Було встановлено, що 4-тижнева терапія розацеа 0,5% гелем брімонідин тартрату, який застосовувався один раз на день, за своєю ефективністю значно перевершувала ефект плацебо.47 Помітне покращення спостерігалося вже через 30 хвилин після першого застосування. Побічні ефекти були помірно виражені, найчастіше у вигляді еритеми (5,1%), свербежу (5,0%), подразнення шкіри (1,2%) та погіршення перебігу розацеа (1,1%). Не було зафіксовано випадків тахіфілаксії чи синдрому відміни. Крім того, нещодавно були опубліковані дані 12-місячного багатоцентрового відкритого дослідження про стійку ефективність брімонідину без розвитку тахіфілаксії при тривалому лікуванні помірної і тяжкої еритеми розацеа.48
Нещодавно завершено другу фазу клінічних випробувань іншого перспективного агоніста α-адренорецепторів – оксиметазоліну.49 Очікується, що результати будуть доступні найближчим часом.
Інші методи лікування
Топічний 10% сульфацетамід натрію з 5% вмістом сірки
Топічний 10% сульфацетамід натрію з 5% вмістом сірки використовується понад 50 років для лікування розацеа, хоча механізм його дії залишається недостатньо вивченим. В 8-тижневому дослідженні було показано, що сульфацетамід натрію 10% з 5% вмістом сірки значно зменшував запальні ураження (78% проти 18%) і вираженість еритеми обличчя (83% проти 31%, р<0,001) порівняно з плацебо (основа препарату).50 Однак дослідження, що оцінюють цю терапію, обмежені і загалом мають низьку якість.28
Пероральний ізотретиноїн
Пероральний ізотретиноїн може застосовуватися поза основним показанням для лікування тяжких або стійких випадків папульозно-пустульозної розацеа, а також здатний уповільнювати або зупиняти прогресування ринофіми.
У 12-тижневому дослідженні, яке порівнювало ефективність різних доз ізотретиноїну, доксицикліну та плацебо при лікуванні розацеа, ізотретиноїн у дозі 0,3 мг/кг продемонстрував ефективність, не нижчу за доксициклін, і добре переносився.51 Однак ізотретиноїн слід призначати тільки при ретельному моніторингу, особливо у жінок репродуктивного віку, оскільки відповідна контрацептивна стратегія є надзвичайно важливою через його тератогенний потенціал.
Лазерні та світлові методи лікування
Лазерні та світлові методи лікування вже багато років успішно застосовуються для усунення судинних проявів розацеа. У рандомізованих дослідженнях імпульсний лазер на барвниках та методи інтенсивного імпульсного світла показали однакову ефективність у зменшенні еритеми та телеангіектазій у пацієнтів з еритематозно-телеангіектазічною розацеа.52
Топічний пероксид бензоїлу та кліндаміцин
У 12-тижневому клінічному дослідженні53 пацієнтам з розацеа призначали 5% топічний пероксид бензоїлу та 1% кліндаміцин один раз на день. Було продемонстровано значне зменшення папульозно-пустульозних висипань у порівнянні з плацебо, що отримували основу препарату (71,3% проти 19,3%, р=0,0056). Побічні ефекти були незначними, найчастіше зустрічалися відчуття печіння та свербіж.
Топічний пімекролімус
В недавньому дослідженні було продемонстровано ефективність і хорошу переносимість 1% крему пімекролімусу для лікування розацеа.54 Побічні ефекти були короткочасними та мали легкий характер, включаючи місцеве печіння, свербіж, сухість та поколювання.
Висновок
Існує багато варіантів лікування розацеа, проте лише деякі препарати мають високу доказову базу. Якщо це можливо, приймаючи терапевтичні рішення щодо лікування розацеа, лікар має керуватися високим рівнем доказів, а також враховувати такі фактори, як підтип розацеа, тяжкість дерматозу, очікування від лікування, переносимість, вартість і попередній досвід терапії.
Топічні азелаїнова кислота та метронідазол вважаються безпечними й ефективними засобами першої лінії. Використання субантибактеріальних доз доксицикліну має вагомі наукові підтвердження і може застосовуватися для лікування помірних і тяжких форм папульозно-пустульозної або очної розацеа. Низькі дози ізотретиноїну або хірургічні втручання можуть бути доцільними для лікування фіматозного типу розацеа. Лазерна терапія може успішно використовуватися для усунення телеангіектазій. Новий метод лікування — брімонідин — призначений для усунення дифузної еритеми обличчя при розацеа. Комплексний підхід до лікування також має включати немедикаментозні стратегії, спрямовані на покращення якості життя, запобігання загостренням, належний щоденний догляд за шкірою, камуфляжну терапію та фотозахист.
Необхідні подальші дослідження для оцінки ефективності комбінованої терапії, ізотретиноїну, сульфацетаміду та нових препаратів у порівнянні зі стандартними методами лікування.
Література:
- Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004 Sep; 51(3):327-41; quiz 42-4.
- Powell FC. Clinical practice. Rosacea. N Engl J Med. 2005 Feb 24; 352(8):793-803.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004 Jun; 50(6):907-12.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002 Apr; 46(4):584-7.
- Gupta AK, Chaudhry MM. Rosacea and its management: an overview. J Eur Acad Dermatol Venereol. 2005 May; 19(3):273-85.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997 Mar; 90(3):144-50.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013 Dec; 69(6 Suppl 1):S27-35.
- Gallo R, Drago F, Paolino S, et al. Rosacea treatments: What’s new and what’s on the horizon? Am J Clin Dermatol. 2010; 11(5):299-303.
- Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013 Dec; 69(6 Suppl 1):S57-65.
- Aksoy B, Altaykan-Hapa A, Egemen D, et al. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol. 2010 Oct; 163(4):719-25.
- Wolf JE, Jr., Del Rosso JQ. The CLEAR trial: results of a large community-based study of metronidazole gel in rosacea. Cutis. 2007 Jan; 79(1):73-80.
- Gooderham M. Rosacea and its topical management. Skin Therapy Lett. 2009 Feb; 14(2):1-3.
- Nielsen PG. Treatment of rosacea with 1% metronidazole cream. A doubleblind study. Br J Dermatol. 1983 Mar; 108(3):327-32.
- Narayanan S, Hunerbein A, Getie M, et al. Scavenging properties of metronidazole on free oxygen radicals in a skin lipid model system. J Pharm Pharmacol. 2007 Aug; 59(8):1125-30.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998 Jun; 134(6):679-83.
- Breneman DL, Stewart D, Hevia O, et al. A double-blind, multicenter clinical trial comparing efficacy of once-daily metronidazole 1 percent cream to vehicle in patients with rosacea. Cutis. 1998 Jan; 61(1):44-7.
- Bleicher PA, Charles JH, Sober AJ. Topical metronidazole therapy for rosacea. Arch Dermatol. 1987 May; 123(5):609-14.
- Rowe-Jones DC, Peel AL, Kingston RD, et al. Single dose cefotaxime plus metronidazole versus three dose cefuroxime plus metronidazole as prophylaxis against wound infection in colorectal surgery: multicentre prospective randomised study. BMJ. 1990 Jan 6; 300(6716):18-22.
- Yoo J, Reid DC, Kimball AB. Metronidazole in the treatment of rosacea: do formulation, dosing, and concentration matter? J Drugs Dermatol. 2006 Apr; 5(4):317-9.
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999 Jun;40(6 Pt 1):961-5.
- Dahl MV, Jarratt M, Kaplan D, et al. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001 Nov; 45(5):723-30.
- Jorizzo JL, Lebwohl M, Tobey RE. The efficacy of metronidazole 1% cream once daily compared with metronidazole 1% cream twice daily and their vehicles in rosacea: a double-blind clinical trial. J Am Acad Dermatol. 1998 Sep; 39(3):502-4.
- Tan JK, Girard C, Krol A, et al. Randomized placebo-controlled trial of metronidazole 1% cream with sunscreen SPF 15 in treatment of rosacea. J Cutan Med Surg. 2002 Nov-Dec; 6(6):529-34.
- Fitton A, Goa KL. Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs. 1991 May; 41(5):780-98.
- Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003 Jun; 48(6):836-45.
- Carmichael AJ, Marks R, Graupe KA, et al. Topical azelaic acid in the treatment of rosacea. J Dermatolog Treat. 1993; 4(suppl):S19-S22.
- Bjerke R, Fyrand O, Graupe K. Double-blind comparison of azelaic acid 20% cream and its vehicle in treatment of papulo-pustular rosacea. Acta Derm Venereol. 1999 Nov; 79(6):456-9.
- van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011(3):CD003262.
- Colon LE, Johnson LA, Gottschalk RW. Cumulative irritation potential among metronidazole gel 1%, metronidazole gel 0,75%, and azelaic acid gel 15%. Cutis. 2007 Apr; 79(4):317-21.
- Thiboutot DM, Fleischer AB, Jr., Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008 Jun; 7(6):541-6.
- Pelle MT, Crawford GH, James WD. Rosacea: II. Therapy. J Am Acad Dermatol. 2004 Oct; 51(4):499-512; quiz 3-4.
- Maibach H. Second-generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991 Nov; 48(5):411-7.
- Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007 May; 56(5):791-802.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008 Jun; 7(6):573-6.
- Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 2005 Sep; 27(9):1329-42.
- Sanchez J, Somolinos AL, Almodovar PI, et al. A randomized, double-blind, placebo-controlled trial of the combined effect of doxycycline hyclate 20-mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. J Am Acad Dermatol. 2005 Nov; 53(5):791-7.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007 Sep; 157(3): 474-81.
- O’Reilly N, Menezes N, Kavanagh K. Positive correlation between serum immunoreactivity to Demodex-associated Bacillus proteins and erythematotelangiectatic rosacea. Br J Dermatol. 2012 Nov; 167(5):1032-6.
- Allen KJ, Davis CL, Billings SD, et al. Recalcitrant papulopustular rosacea in an immunocompetent patient responding to combination therapy with oral ivermectin and topical permethrin. Cutis. 2007 Aug; 80(2):149-51.
- Forstinger C, Kittler H, Binder M. Treatment of rosacea-like demodicidosis with oral ivermectin and topical permethrin cream. J Am Acad Dermatol. 1999 Nov; 41(5 Pt 1):775-7.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004 Aug; 31(8):618-26.
- Galderma. Randomized, double-blind, parallel-group, vehicle-controlled, dose-finding study investigating the pharmacodynamics and safety of three concentrations of CD07805/47 topical gel (0,07%, 0,18%, and 0,50%), applied in subjects with moderate to severe erythematotelangiectatic rosacea. In: ClinicalTrials.gov, Identifier: NCT00989014. Last updated September 19, 2013. Available at: http://clinicaltrials.gov/ct2/show/NCT00989014. Accessed: March 9, 2014.
- Galderma. A phase 3 randomized, double blind, 12 week vehicle controlled, parallel group study assessing the efficacy and safety of CD5024 1% cream versus vehicle cream in subjects with papulopustular rosacea, followed by a 40 week investigator blinded extension comparing the long term safety of CD5024 1% cream versus azelaic Acid 15% gel. In: ClinicalTials.gov, Identifier: NCT01493687. Last updated January 27, 2014. Available at: http://clinicaltrials.gov/ct2/show/NCT01493687?term=NCT01493687&rank=1. Accessed: March 10, 2014.
- Galderma. Efficacy and safety of CD5024 1% cream versus metronidazole 0.75% cream in subjects with papulopustular rosacea over 16 weeks treatment, followed by a 36-week extension period (ATTRACT).In: ClinicalTrials.gov, Identifier: NCT01493947. Last updated December 20, 2013. Available at: http://clinicaltrials.gov/ct2/show/NCT01493947?term=NCT01493947&rank=1. Accessed: March 9, 2014.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 2: the central role, evaluation, and medical management of diffuse and persistent facial erythema of rosacea. J Clin Aesthet Dermatol. 2012 Mar; 5(3):26-36.
- Rahman MQ, Ramaesh K, Montgomery DM. Brimonidine for glaucoma. Expert Opin Drug Saf. 2010 May; 9(3):483-91.
- Fowler J. Jr., Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehiclecontrolled pivotal studies. J Drugs Dermatol. 2013 Jun 1; 12(6):650-6.
- Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014 Jan; 13(1):56-61.
- Allergan. Safety and tolerability of AGN-199201 in patients with erythema associated with rosacea. In: Clinical Trials.gov, Identifier: NCT01579084. Last updated September 12, 2013. Available at: http://clinicaltrials.gov/ct2/show/NCT01579084?term=NCT01579084&rank=1. Accessed: March 10, 2014.
- Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatolog Treat. 1997; 8(2):79-85.
- Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea — doxycycline- and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010 Jul; 8(7):505-15.
- Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009 Jun; 35(6):920-8.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehiclecontrolled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004 May; 43(5):381-7.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011 Dec; 38(12):1135-9.
ЧАНГ Б., КУР’ЯН А. — Університет Альберта, Едмонтон (Канада)
БАРАНКІН Б. — Дерматологічний центр Торонто (Канада)