AbbVie Submits Biologics License Application to U.S. FDA of a new botulinum toxin type E
AbbVie Submits Biologics License Application to U.S. FDA for TrenibotulinumtoxinE (TrenibotE) for the Treatment of Glabellar Lines.
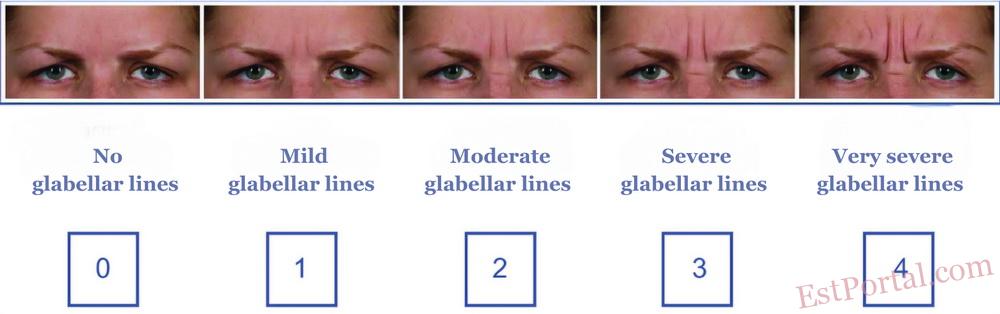
- TrenibotE is a first-in-class botulinum neurotoxin serotype E characterized by a rapid onset of action as early as 8 hours after administration (earliest assessment time) and shorter duration of effect of 2-3 weeks.
- If approved, TrenibotE will be the first neurotoxin of its kind available to patients.
- Submission is supported by data from over 2,100 patients treated with TrenibotE throughout the clinical program.
April 24, 2025 — North Chicago, Illinois /PRNewswire/ — Allergan Aesthetics, an AbbVie company, announced the submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for TrenibotulinumtoxinE (TrenibotE) — a novel botulinum neurotoxin serotype E intended for the treatment of moderate to severe glabellar lines.
If approved, TrenibotE would become the first-ever botulinum toxin serotype E to be used in aesthetic medicine. It offers a rapid onset of action (8 hours) and a short clinical duration of 2-3 weeks. This unique pharmacological profile makes TrenibotE a compelling option for patients seeking to try injectable neurotoxins with the ability to assess results without long-lasting changes to appearance.
“The submission provides evidence of TrenibotE's differentiated clinical profile to offer patients an opportunity to experience a faster onset and shorter treatment duration as an introduction to a neurotoxin,” said Darin Messina, Ph.D., senior vice president, aesthetics R&D, AbbVie. “TrenibotE has the potential to transform the aesthetic toxin treatment landscape for new patients interested in the facial aesthetics category.”
One of the main barriers for new patients considering neurotoxin treatment is the fear of looking “unnatural” or “frozen.” Thanks to its rapid onset and limited duration, TrenibotE may serve as an ideal entry-point neurotoxin for individuals exploring aesthetic injections for the first time.
Clinical Program Overview
The FDA submission is supported by data from over 2,100 patients enrolled in AbbVie’s clinical program, including:
- Two pivotal Phase 3 studies (M21-500 and M21-508)
- One open-label Phase 3 safety study (M21-509)
These trials evaluated the efficacy and safety of TrenibotE in patients with moderate to severe glabellar lines. The majority of participants were either neurotoxin-naïve or had prior experience with botulinum toxins.
All primary and secondary endpoints were met. According to the Facial Wrinkle Scale (FWS), statistically significant improvements in wrinkle severity were observed as early as Day 7 post-injection (p<0.0001). Clinical effect was detected as early as 8 hours after administration — the earliest time point evaluated — and lasted 2-3 weeks.
Treatment-emergent adverse events were similar to placebo across both single and repeat treatments. The safety profile remained consistent and favorable.
“Concern about an unnatural outcome remains a significant barrier for many patients considering medical aesthetics treatment,” said Cheryl Burgess, MD, FAAD, lead clinical investigator for one of the Phase 3 studies. “Treatment with a product offering rapid onset of effect and short duration of action could help address this barrier and empower confidence for patients exploring their aesthetics treatment journey with innovation from the makers of BOTOX® Cosmetic.”
Impact on the Aesthetic Medicine Industry
Allergan Aesthetics, an AbbVie company, previously announced positive topline results from both Phase 3 trials, reinforcing TrenibotE’s status as a first-in-class molecule. With its novel pharmacological properties, TrenibotE may complement existing long-acting botulinum toxins, such as type A products, by offering a more customizable approach to toxin selection based on patient preferences for onset speed and duration.
“BoNT/E is a next-generation, short-acting neurotoxin, the first of its kind. These results clearly demonstrate its potential to bring true innovation to aesthetic medicine,” added Dr. Messina. “This data marks meaningful progress in advancing our next-generation aesthetics toxin pipeline.”
This article was developed based on official press materials from AbbVie and Allergan Aesthetics. For more updates on next-generation neurotoxins, follow ESTportal.com
Nataliya CHAYKA – Editor of ESTportal, Aesthetic Doctor